
This activity is provided by Med Learning Group.
This activity is supported by an educational grant from Lilly.
Copyright © 2024 Med Learning Group. Built by Divigner. All Rights Reserved.
There is no cure for Alzheimers disease (AD), however, disease progression can be altered with non-pharmacologic and pharmacologic treatment options.1,2 AD is a neurodegenerative condition that typically gets worse over time, and treatments are generally most effective in the early stage of the disease process.3
Non-pharmacologic interventions do not change the underlying disease course, however, are often implemented to help maintain or improve cognitive function as well as reduce behavioral symptoms.2 Such intervention can include cognitive stimulation, music-based therapy, psychological treatment (ie, cognitive behavioral therapy), physical therapy/exercise, and a healthy diet.2,3 Safety, effectiveness and purity of alternative therapies/supplements is largely unknown, although trials evaluating the effectiveness of some “medical foods”, such as medium-chain triglycerides, are being conducted.4
Mainstays for pharmacologic therapy focus on either temporarily managing AD symptoms or treating the underlying cause of disease.5,6 In terms of pharmacologic therapy, to date seven drugs have received approval from the US FDA for the treatment of AD. These include three acetylcholinesterse inhibitors (donepezil, rivastigmine, and galantamine), one N-methyl-D-aspratate [NMDA] antagonist (memantine), one combination medications (memantine plus donepezil), and three disease modifying therapies (aducanumab, donanemab and lecanemab).2,23
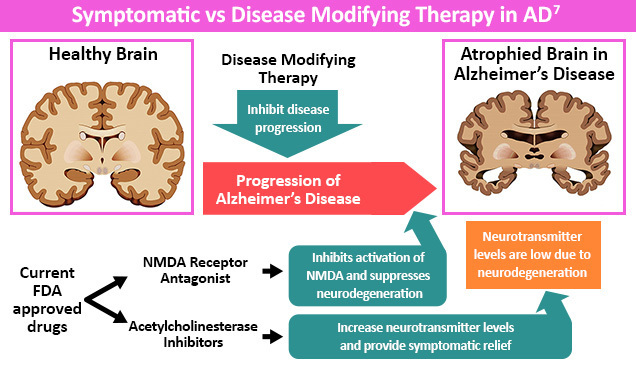
People with AD have diminished cholinergic function due to decreased acetylcholine synthesis.1 Cholinesterase inhibitors, like donepezil, rivastigmine, and galantamine, can increase cholinergic transmission leading to an improvement in AD symptoms.1
People with all stages of AD may benefit from a trial of cholinesterase inhibitors as they may show a small benefit in cognition, neuropsychiatric symptoms, and activities of daily living (ADLs).1 However, results may be variable, as studies suggest that no observable benefit is seen in up to 30%-50% of people with AD.1 On the other end of the spectrum, up to 20% of people may see above-average improvement in symptoms.1 Side effects of cholinesterase inhibitors include nausea, vomiting, diarrhea, sleep disturbances, bradycardia, cardiac conduction defects, and syncope.3 Additionally, avoidance of tricyclic antidepressants for treatment of anxiety, depression and psychosis is key due to their anticholinergic activity.3
Memantine is another therapeutic option for AD that can be given as monotherapy or in combination with cholinesterase inhibitors.3 This agent acts by blocking pathologic stimulation of NMDA receptors by neurotransmitter glutamate and slowing intracellular calcium accumulation.1,2,3 This distinct mechanism of action is considered neuroprotective against glutamate and may restore physiologic neuronal function.1,2 Studies also suggest memantine may give patients a small improvement in cognition, dementia, and quality of life.1 Common side effects of memantine include dizziness, body aches, headache, and constipation.3
The United States Food and Drug Administration (FDA) has approved aducanumab (2021) and lecanemab (2023) through an accelerated approval pathway, and full approval of donanemab (2024).8,9,23 Aducanumab is a recombinant monoclonal antibody targeting amyloid-beta (EMERGE and ENGAGE trials) approved for people with mild cognitive impairment (MCI) and the mild dementia stage of AD, but is recommended to limit use to those with documented amyloid pathology on amyloid positron emission tomography (PET) scans.1,2,7,10 Aducanumab is associated with an increased risk of amyloid-related imaging abnormities (ARIA) which can be an indicator of brain swelling; post-approval trials are required to verify clinical benefit.1, 2 However, it should be noted that aducanumab is being discontinued in 2024. Although no longer available through clinical trial, the manufacturer, Biogen has stated that patients receiving aducanumab via prescription from their treating physicians will have it available until Nov. 1, 2024.7 Lecanemab is another monoclonal antibody targeting β-amyloid protofibrils (CLARITY-AD), with approved indications of use in those with MCI and the mild dementia stage of AD, following confirmed presence of amyloid-beta pathology.5,11 ARIA events have also been associated with lecanemab; post approval trials are required to verify clinical benefit.5
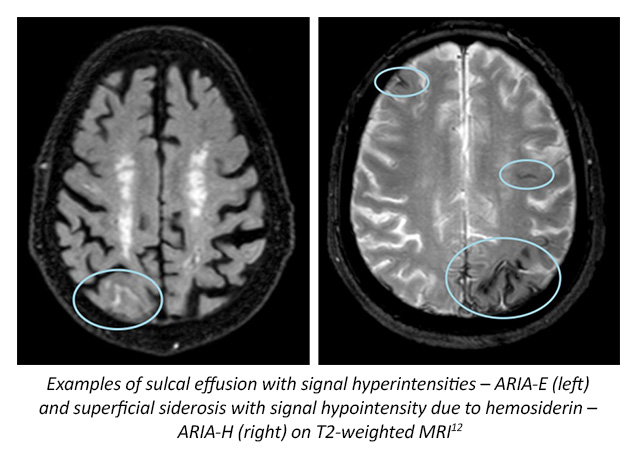
Donanemab, also a monoclonal antibody targeting the N-terminal pyroglutamate epitope, a modified form of amyloid-beta present only in established plaques.14,15 Clinical trials suggest that this new medication not only reduces amyloid plaques as measured by 18 F-florbetapir uptake on PET, but also lowers levels of plasma tau biomarkers (TRAILBLAZER-ALZ and TRAILBLAZER-ALZ2 trials).14,15 Donanemab has also been shown to have less cognitive and functional decline over placebo.14 ARIA has also been observed with use of donanemab in the clinical trial setting.14
At the present time a total of 36 agents are being assessed in 55 different AD trials, among which disease modifying agents comprise 67%.13 Another theraputic in the research pipeline includes gantenerumab, a monoclonal antibody targeting amyloid-beta.16 Gantenerumab binds aggregated amyloid beta plaques by phagocytosis, and also showed a robust decrease in amyloid plaques after 2 years of treatment.16
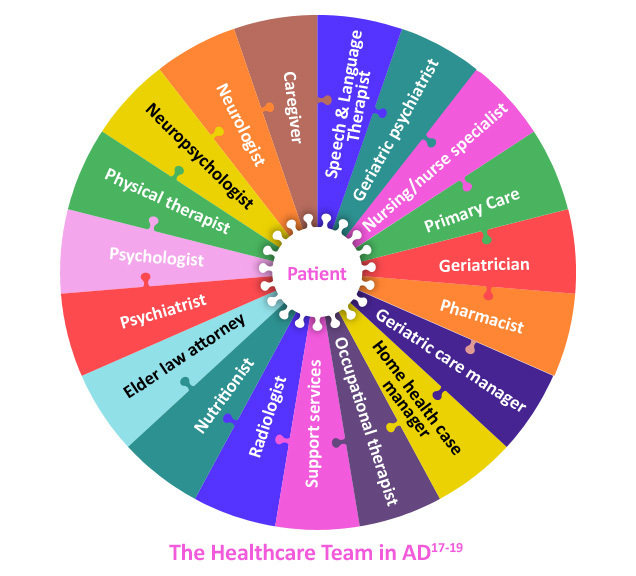
Management goals for AD include strategizing to optimize dementia outcomes, focusing on lessening symptoms and maintaining quality of life through maximizing function, cognition, behavior and safety.20 A multidisciplinary approach and collaborative care model focuses on long-term, systematic approaches for management of chronic conditions like AD, possibly utilizing cost-efficient strategies to improve health outcomes and shared decision-making for individualized treatment.21 This team can help facilitate adaptation to cognitive and behavioral changes with disease progression as well as refer for participation in dementia-related clinical trials.20,22 Nonmedical issues related to long-term care planning for changing needs over the course of AD can also be discussed, such as establishing a healthcare power of attorney, creating a living will, determining end-of-life care preferences and reviewing finances.18
Click here to view and download a cognitive impairment care planning toolkit provided by the Alzheimer’s Association®.
All URLs accessed February 14, 2024.

This activity is provided by Med Learning Group.
This activity is supported by an educational grant from Lilly.
Copyright © 2024 Med Learning Group. Built by Divigner. All Rights Reserved.
Esta actividad es proporcionada por Med Learning Group. Esta actividad es co-proporcionada por Ultimate Medical Academy / Complete Conference Management (CCM).
Esta actividad está respaldada por una beca de educación médica independiente de Regeneron Pharmaceuticals, Inc.

Copyright © 2019 Med Learning Group. Construido por Divigner. Reservados todos los derechos.

Chief Medical Officer
Frank C. and Lynn Scaduto MIND Institute and Behavioral Health
Miami Jewish Health
Miami, FL

Associate Dean of Alzheimer’s Disease Research
Indiana University Distinguished Professor
Barbara and Peer Baekgaard Professor of Alzheimer's Disease Research
Professor in Neurology, Radiology, Medical and Molecular Genetics
Indiana University School of Medicine
Department of Neurology
Indianapolis, IN

Chief Medical Officer, Banner Research
Banner Alzheimer’s and Research Institutes
Pheonix, Sun City, and Tucson, AZ
Director, Banner Sun Health Research Institute
Sun City, AZ

Program Director, AdventHealth Geriatric Fellowship
Winter Park, FL

Director, Massachusetts General Hospital
Frontotemporal Disorders Unit
Professor of Neurology, Harvard Medical School
Boston, MA

Professor of Psychiatry and Human Behavior
Sidney Kimmel Medical College
Co-Director, Comprehensive Alzheimer’s Center
Vickie & Jack Farber Institute for Neuroscience
Thomas Jefferson University
Philadelphia, PA

Clinical Professor of Medicine
Tufts University School of Medicine
Clinical professor, Department of Public Health and Community Medicine, Tufts University
Chief, Geriatrics Service, Tufts Medical Center
Senior Physician, Pratt Diagnostic Center
Dean ex officio, Office of International Affairs, Tufts University School of Medicine
Boston, MA

Professor of Neurology
University of Miami Miller School of Medicine
Miami, FL

Professor and Director
Division of Memory Disorders and Behavioral Neurology
Department of Neurology
Heersink School of Medicine
University of Alabama at Birmingham
Birmingham, AL

Henry & Amelia Nasrallah Endowed Professor
Director of Geriatric Psychiatry
Department of Psychiatry & Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, MO

Director of Geriatric Cognitive Health
Pacific Neuroscience Institute
Santa Monica, CA
Adjunct Professor
USC Leonard Davis School of Gerontology
Los Angeles, CA

President, Kerwin Medical Center
Chief, Geriatric Medicine, Texas Health Presbyterian Hospital
Dallas, TX

Assistant Professor
UT Southwestern Neurology
Dallas, TX

Director, CurePSP Center of Care for PSP, CBD, and MSA
Assistant Professor of Neurology
Alzheimer's and Parkinson's Disease Centers
Baylor College of Medicine
Houston, TX

Founding Director Ray Dolby Brain Health Center
San Francisco, CA

Assistant Professor of Neurology, Harvard Medical School
Center for Alzheimer Research and Treatment
Brigham and Women's Hospital
Frontotemporal Disorders Unit
Massachusetts General Hospital
Boston, MA

Director, Division of Geriatrics
Co-director, Center for Memory Loss and Brain Health
Hackensack University Medical Center
Hackensack, NJ

The Saunders Family Chair and Professor of Neurology
Director of the Center for Molecular Integrative Neuroresilience,
Professor of Psychiatry and Neuroscience
Professor of Geriatrics and Adult Development
Department of Neurology and Friedman Brain Institute
Icahn School of Medicine at Mount Sinai
New York, NY

Chief Medical Officer
Advocate Good Samaritan Hospital
Downers Grove, IL

Moreno Family Chair for Alzheimer’s Research
Vice Chairman for Research and Professor
Department of Neurology
Barrow Neurological Institute
Phoenix, AZ

Rick McCord Professor in Neurology
Umphrey Family Professor of Neurodegenerative Diseases
Director, Neurocognitive Disorders Center
Director, Neurocognitive Disorders Fellowship
McGovern Medical School at UTHealth Houston
Houston, TX

Professor of Family and Community Medicine
Sidney Kimmel Medical College
Thomas Jefferson University
Philadelphia, PA

Professor of Neurology
Director of the Memory Disorders Program
Georgetown University
Washington, DC

Health Sciences Clinical Professor
UC Irvine Department of Family Medicine
Director, UCI Program in Medical Education for the Latino Community
University of California Irvine
Irvine, CA

John R. Ellis Distinguished Chair of Pharmacy Practice
Professor of Clinical Sciences
Director, Drake Drug Information Center
Drake University College of Pharmacy and Health Sciences
Internal Medicine Clinical Pharmacist
Iowa Methodist Medical Center
Des Moines, IA

Professor of Neurology
Director, Penn Alzheimer’s Disease Research Center
University of Pennsylvania
Philadelphia, PA