
This activity is provided by Med Learning Group.
This activity is supported by an educational grant from Lilly.
Copyright © 2024 Med Learning Group. Built by Divigner. All Rights Reserved.
Alzheimer’s disease (AD) is a neurodegenerative condition, characterized by the accumulation of extra-neuron beta-amyloid protein (plaques) and intra-neuron tau protein (tangles) in the brain.1 The stages of AD are classified by degree of cognitive impairment, ranging from no AD symptoms in the preclinical form to severe symptoms in later stages.1,2
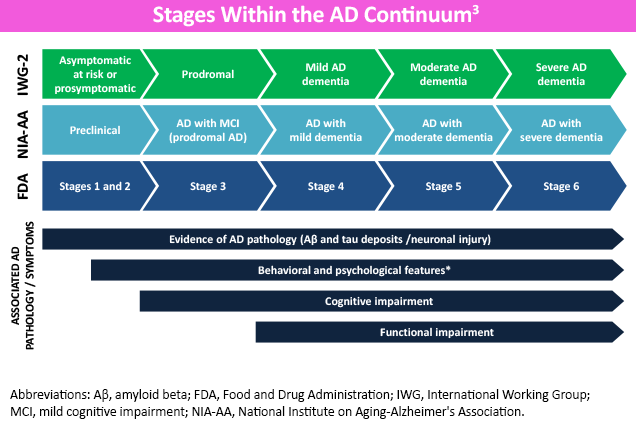
One of the earliest and most common symptoms of AD is memory loss, specifically “recent” memory impairment with relative sparing of long-term memory.2,4 Patients with AD may eventually develop difficulties with problem-solving, judgment, organization and executive function, and subsequently impaired multitasking and abstract thinking.2 With disease progression arises the inability to complete tasks and reduced insight, potentially impacting capability to safely perform activities like driving.4
In later stages, language disorders and trouble with visuospatial skills arise.2 Moderate-to-late stages of AD may also demonstrate neuropsychiatric symptoms of apathy, lack of inhibition, psychosis, agitation, social withdrawal, and wandering, and is associated with a more rapid decline if present early.2,4 In end-stage AD, difficulties with verbal communication and movement can result in being bed-bound and incontinent, with total dependence on caregivers.1,2
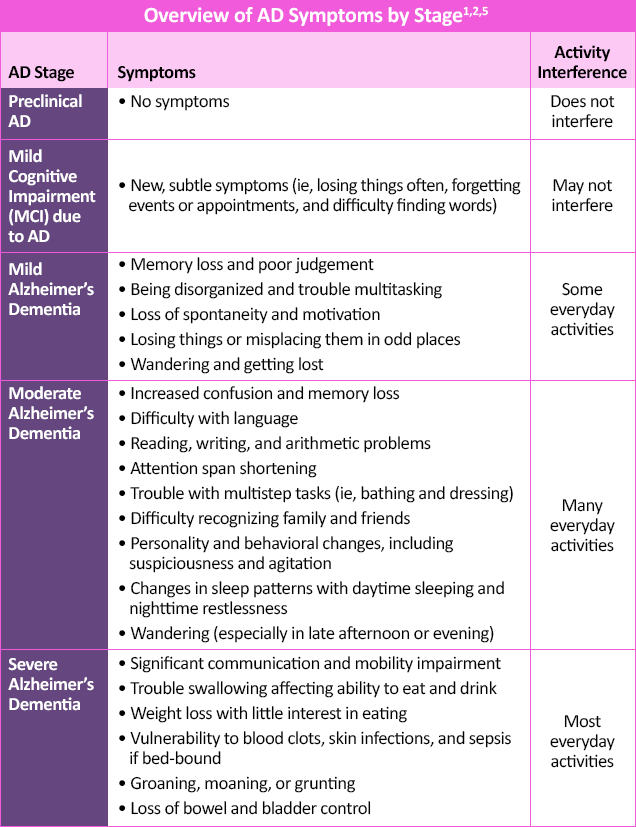
Since AD generally worsens over time, early diagnosis benefits both the patients and their caregivers.2 It allows them to address modifiable risk factors that may delay cognitive decline, start treatment or enroll in clinical trials before severe stages have set in, and potentially preserve daily functioning for longer.1 Early diagnosis of AD also gives patients and caregivers additional time to learn how to manage behavioral symptoms, discuss future planning and personal/financial issues, address safety concerns, and establish a support network.1
The diagnosis of AD starts by obtaining an accurate history, especially from family members and caregivers.2 A complete physical exam, including neurologic findings, helps rule out other potential causes of dementia.2 Recent studies suggest that anosmia may be an early marker of AD, but more research is needed and this is not a widely used diagnostic finding.6
Detailed cognitive, neurologic, and psychological evaluation can provide a great deal of information about preservation or loss of independent function, as well as presence/types of neuropsychiatric symptoms.4 Mental status exams, especially those evaluating concentration, attention, memory, language, praxis, and visuospatial and executive functioning, can help document the presence and stage of dementia.2,4 Furthermore, formal neuropsychological testing and cognitive assessments can not only establish a dementia baseline, but also help distinguish among other forms of dementia (pseudodementia, Lewy body dementia, vascular dementia, and frontotemporal lobar degeneration) as well as assess competencies for performing potentially dangerous tasks.4 Neuropsychological testing is also the most reliable method for detecting MCI.2 Click here to view and download a cognitive assessment toolkit provided by the Alzheimer’s Association®.
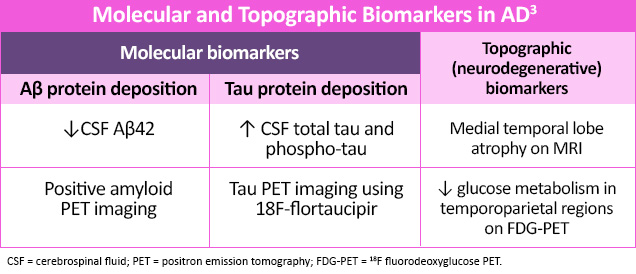
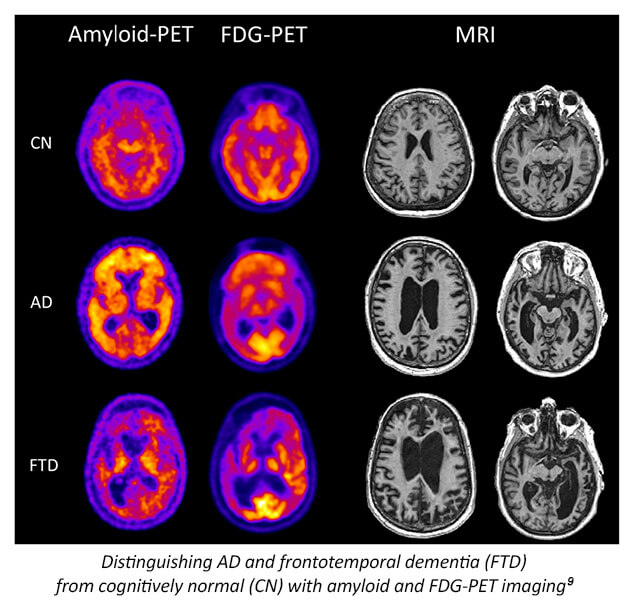
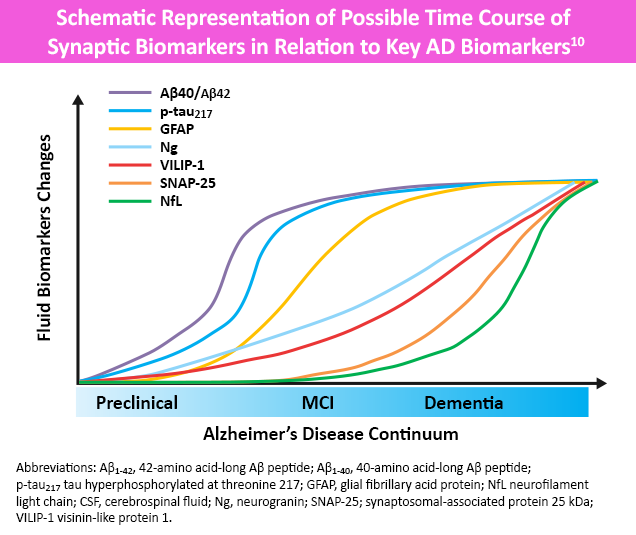
While not yet recommended for routine diagnostic use, several serologic, plasma and advanced imaging biomarkers are being researched to help support an AD diagnosis.4,7 Molecular biomarkers identify specific protein deposits in the brain, like amyloid found in amyloid plaques or tau proteins found in neurofibrillary tangles.4 Topographic (neurodegenerative) biomarkers help locate regional pathologic brain changes associated with AD.3 Neuroimaging, including magnetic resonance imaging (MRI) and functional brain imaging (eg, amyloid/tau PET and single-photon emission computed tomography [SPECT]) may also help rule out other potential causes of dementia.2,4 More recently, focus has switched to pTau217, i.e. tau phosphorylated at amino acid 217, as this biomarker has consistently shown high performance in differentiating AD from other neurodegenerative disorders and in detecting AD pathology in patients with MCI.8
The most common comorbidities for AD are type 2 diabetes, cardiovascular disease, depression, and inflammatory bowel disease. A key factor in these common chronic diseases is the presence of inflammation. Research is ongoing as to whether inflammation is a cause or effect of AD; likely, it has a bidirectional influence on this neurodegenerative disorder.
Regardless, the presence of these comorbidities noticed in AD patients may have significant implications for the treatment and prognosis of AD. For instance, both diabetes and depression are associated with a worse prognosis in people with AD. Similarly, certain lifestyle factors, like diet and exercise, may also have an impact on cognitive function.
All URLs accessed on February 14, 2024

This activity is provided by Med Learning Group.
This activity is supported by an educational grant from Lilly.
Copyright © 2024 Med Learning Group. Built by Divigner. All Rights Reserved.
Esta actividad es proporcionada por Med Learning Group. Esta actividad es co-proporcionada por Ultimate Medical Academy / Complete Conference Management (CCM).
Esta actividad está respaldada por una beca de educación médica independiente de Regeneron Pharmaceuticals, Inc.

Copyright © 2019 Med Learning Group. Construido por Divigner. Reservados todos los derechos.

Chief Medical Officer
Frank C. and Lynn Scaduto MIND Institute and Behavioral Health
Miami Jewish Health
Miami, FL

Associate Dean of Alzheimer’s Disease Research
Indiana University Distinguished Professor
Barbara and Peer Baekgaard Professor of Alzheimer's Disease Research
Professor in Neurology, Radiology, Medical and Molecular Genetics
Indiana University School of Medicine
Department of Neurology
Indianapolis, IN

Chief Medical Officer, Banner Research
Banner Alzheimer’s and Research Institutes
Pheonix, Sun City, and Tucson, AZ
Director, Banner Sun Health Research Institute
Sun City, AZ

Program Director, AdventHealth Geriatric Fellowship
Winter Park, FL

Director, Massachusetts General Hospital
Frontotemporal Disorders Unit
Professor of Neurology, Harvard Medical School
Boston, MA

Professor of Psychiatry and Human Behavior
Sidney Kimmel Medical College
Co-Director, Comprehensive Alzheimer’s Center
Vickie & Jack Farber Institute for Neuroscience
Thomas Jefferson University
Philadelphia, PA

Clinical Professor of Medicine
Tufts University School of Medicine
Clinical professor, Department of Public Health and Community Medicine, Tufts University
Chief, Geriatrics Service, Tufts Medical Center
Senior Physician, Pratt Diagnostic Center
Dean ex officio, Office of International Affairs, Tufts University School of Medicine
Boston, MA

Professor of Neurology
University of Miami Miller School of Medicine
Miami, FL

Professor and Director
Division of Memory Disorders and Behavioral Neurology
Department of Neurology
Heersink School of Medicine
University of Alabama at Birmingham
Birmingham, AL

Henry & Amelia Nasrallah Endowed Professor
Director of Geriatric Psychiatry
Department of Psychiatry & Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, MO

Director of Geriatric Cognitive Health
Pacific Neuroscience Institute
Santa Monica, CA
Adjunct Professor
USC Leonard Davis School of Gerontology
Los Angeles, CA

President, Kerwin Medical Center
Chief, Geriatric Medicine, Texas Health Presbyterian Hospital
Dallas, TX

Assistant Professor
UT Southwestern Neurology
Dallas, TX

Director, CurePSP Center of Care for PSP, CBD, and MSA
Assistant Professor of Neurology
Alzheimer's and Parkinson's Disease Centers
Baylor College of Medicine
Houston, TX

Founding Director Ray Dolby Brain Health Center
San Francisco, CA

Assistant Professor of Neurology, Harvard Medical School
Center for Alzheimer Research and Treatment
Brigham and Women's Hospital
Frontotemporal Disorders Unit
Massachusetts General Hospital
Boston, MA

Director, Division of Geriatrics
Co-director, Center for Memory Loss and Brain Health
Hackensack University Medical Center
Hackensack, NJ

The Saunders Family Chair and Professor of Neurology
Director of the Center for Molecular Integrative Neuroresilience,
Professor of Psychiatry and Neuroscience
Professor of Geriatrics and Adult Development
Department of Neurology and Friedman Brain Institute
Icahn School of Medicine at Mount Sinai
New York, NY

Chief Medical Officer
Advocate Good Samaritan Hospital
Downers Grove, IL

Moreno Family Chair for Alzheimer’s Research
Vice Chairman for Research and Professor
Department of Neurology
Barrow Neurological Institute
Phoenix, AZ

Rick McCord Professor in Neurology
Umphrey Family Professor of Neurodegenerative Diseases
Director, Neurocognitive Disorders Center
Director, Neurocognitive Disorders Fellowship
McGovern Medical School at UTHealth Houston
Houston, TX

Professor of Family and Community Medicine
Sidney Kimmel Medical College
Thomas Jefferson University
Philadelphia, PA

Professor of Neurology
Director of the Memory Disorders Program
Georgetown University
Washington, DC

Health Sciences Clinical Professor
UC Irvine Department of Family Medicine
Director, UCI Program in Medical Education for the Latino Community
University of California Irvine
Irvine, CA

John R. Ellis Distinguished Chair of Pharmacy Practice
Professor of Clinical Sciences
Director, Drake Drug Information Center
Drake University College of Pharmacy and Health Sciences
Internal Medicine Clinical Pharmacist
Iowa Methodist Medical Center
Des Moines, IA

Professor of Neurology
Director, Penn Alzheimer’s Disease Research Center
University of Pennsylvania
Philadelphia, PA